Health - Purple Foxy Ladies originally published at Health - Purple Foxy Ladies
 OLATHE, Kan. - October 18, 2022 - (Newswire.com)
OLATHE, Kan. - October 18, 2022 - (Newswire.com)
TVAX Biomedical (TVAX) is a cancer immunotherapy company that is moving into its pivotal glioblastoma study for FDA regulatory approval. In September 2022, TVAX Biomedical received an approximately $2 million 4-year grant from the FDA Office of Orphan Products for its planned glioblastoma study. TVAX Immunotherapy® could fundamentally change the way cancer is treated as T cells can kill all cancer cells, including cancer stem cells, without the toxicity of current treatments. TVAX Biomedical has previously received Fast Track Designation and Orphan Product Designation from the FDA. Dr. Wayne Carter, TVAX Biomedical CEO exclaimed, "We are thrilled to receive this highly competitive grant from the FDA and eager to bring this novel therapy to glioblastoma patients looking for better options!"
TVAX Immunotherapy is a cutting-edge approach to cellular therapy. The individualized therapy first helps the patient develop immunity against their cancer and the unique "neoantigens" that cancer cells produce. Next, T cells are removed from patient's blood and activated in TVAX's specialized manufacturing facility. Patients are then treated with billions of their own "killer" T cells that attack their cancer. Dr. Wayne Carter, TVAX Biomedical's CEO, explains it like this: "TVAX's technology uses 'killer' T cells that function like billions of smart bombs hunting and destroying cancer cells throughout the body. TVAX Immunotherapy has very few side effects because 'killer' T cells kill cancer cells, not normal cells."
This therapy has shown promising results for four-legged friends too. The ability to treat multiple types of cancer prompted TVAX to spin-off its animal health division into Elias Animal Health, an affiliate pursuing similar treatments in dogs. Elias is using the technology to treat osteosarcoma, an incurable bone cancer and is completing their registration study with the USDA.
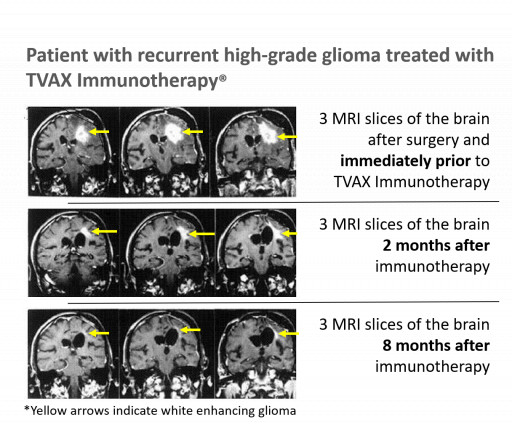
TVAX Immunotherapy has been administered to more than 120 patients and has demonstrated efficacy against several cancers, producing numerous long-lasting complete and partial responses with none of the "off-target" short and long-term toxicity seen with CAR-T cell therapy, checkpoint inhibitors, and chemotherapies.
FDA Fast Track Designation affords many advantages, including a pathway to accelerated marketing approval. TVAX Immunotherapy has dramatically lower manufacturing costs compared to other cellular immunotherapies such as CAR-T therapies.
About TVAX Biomedical
TVAX Biomedical is a clinical stage development company advancing its proprietary personalized T cell-based cancer immunotherapy. The company's lead candidate is supported by positive Phase 2 clinical data, as well as extensive preclinical and Phase 1 safety studies. TVAX plans to initiate a registration study in newly diagnosed glioblastoma patients in 2023. The US brain cancer market is $1.5 billion with many follow-on market opportunities in other types of cancer. As part of its ongoing strategy, the company is seeking investors and corporate alliances to support the development.
For more information please contact: info@tvaxbiomedical.com; 913-492-2221
###
Contact Information:
General Info
Marketing
info@tvaxbiomedical.com
913-492-2221
Press Release Service by Newswire.com
Original Source: TVAX Biomedical Announces Receipt of $2 Million FDA Orphan Products Grant for Glioblastoma Study
Health - Purple Foxy Ladies originally published at Health - Purple Foxy Ladies