ANKARA, Turkey – November 20, 2022 – (Newswire.com)
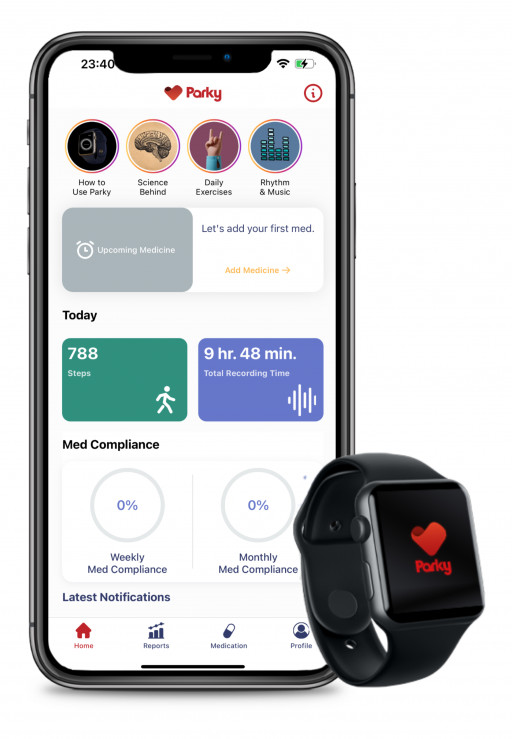
Digital health start-up H2o therapeutics received 510(k) marketing clearance from the U.S. Food and Drug Administration for its prescription mobile app Parky for monitoring Parkinson’s Disease. Parky App monitors symptoms such as tremors and dyskinesia in real-time via the use of the Apple Watch. It is a tool for sharing meaningful and reliable data between patients and medical professionals regarding the course of the disease.
The app leverages the Movement Disorder API, a tool developed by Apple. The API was validated in a study of 343 participants, including longitudinal tracking of 225 participants for up to six months and used the clinical gold standard (MDS-UPDRS) as a reference. Details of the study were published in Science Translational Medicine last year.
10 million patients worldwide live with Parkinson’s Disease, the fastest-growing neurological disorder. Healthcare professionals in this area need reliable feedback and data for optimized treatment plans. Parky App helps medical professionals to develop a clinical profile of the patient while outside of the clinic. The Parky App strengthens the possibility of data-driven, tailored treatment procedures and bridges the gap between real-life and in-clinic settings.
“As a woman-founded, non VC-backed company based in Turkey, receiving our first 510(k) clearance is a huge milestone for us. We believe Parky will bring great value to the Parkinson’s Disease community as an easily scalable and data-driven product,” Yagmur Selin Gulmus, the founder of h2o therapeutics, said in a statement. The company has 2 more digital therapeutics products in the pipeline based on wearable devices and FDA submissions are planned to be completed in 2023.
h2o therapeutics is developing digital therapeutics with a focus on mobile technologies, Augmented Reality (AR), and Artificial Intelligence (AI). h2O intends to make real-time human data become a handy tool for disease management in certain therapeutic areas. Their core understanding of digital therapeutics lies in the power of AI, the robustness of their clinical health research, and high levels of user engagement.
Contact Information:
Yagmur Selin Gulmus
Founder
Hande Erciyes
Operations Manager
Press Release Service
by
Newswire.com
Original Source:
H2o Therapeutics Gets FDA Clearance for Its Apple Watch-Based App for Parkinson’s Disease







