In 2022 Mazda remains as elegant as ever and keeps the fabled Japanese reliability. Today we will cover their latest car lineup that now includes some worthy crossover SUVs. Join us on this informative journey with #AutomotiveTerritoryDailyNews. #1 2023 Mazda CX-60 2023 Mazda CX-60 is the first plug-in hybrid SUV from the brand that belongs […]
In 2022 Mazda remains as elegant as ever and keeps the fabled Japanese reliability. Today we will cover their latest car lineup that now includes some worthy crossover SUVs. Join us on this informative journey with #AutomotiveTerritoryDailyNews. #1 2023 Mazda CX-60 2023 Mazda CX-60 is the first plug-in hybrid SUV from the brand that belongs […]
 Dr. Korb Dr. Korb with her dogs, Charlie and Tucker
Dr. Korb Dr. Korb with her dogs, Charlie and Tucker
CHARLESTON, S.C. – October 11, 2022 – (Newswire.com)
…
 Ambit to showcase its prevalence projection approach, co-developed with Praxis, for rare developmental and epileptic encephalopathies (DEEs) as a Platform Presentation at this year’s annual Child Neurology Society meeting
Ambit to showcase its prevalence projection approach, co-developed with Praxis, for rare developmental and epileptic encephalopathies (DEEs) as a Platform Presentation at this year’s annual Child Neurology Society meeting
CINCINNATI – October 11, 2022 – (Newswire.com)
Ambit®, a data and technology-enabled biopharma services company, today announced that leadership will debut Ambit’s data-driven methodology for projecting prevalence and diagnosis rates for difficult-to-quantify DEEs at the 51st Annual Child Neurology Society Meeting on Oct. 13, 2022. Ambit’s analytics team has developed this methodology in collaboration with Praxis Precision Medicines, Inc. (NASDAQ: PRAX) and the UCSF Weill Institute for Neurosciences and Benioff Children’s Hospitals.
For many rare DEEs, reliable prevalence estimates are not available. With a robust and growing pipeline of precision medicine therapies in rare epilepsy and neurodevelopmental disorders, estimating the prevalence of these conditions with a data-driven methodology can be highly valuable to drug developers, medical device companies and other healthcare decision-makers.
“Ambit’s expertise in a data-driven approach to prevalence projections for rare neurological conditions has allowed Praxis to characterize DEE populations with a high degree of confidence,” said Marcio Souza, president and chief executive officer of Praxis. “As we seek to bring first-in-class, potentially disease-modifying therapies to DEE patients in need, our collaboration with Ambit has been invaluable in increasing our understanding of these patient groups.”
Despite the availability of sponsored genetic testing and increased awareness of the benefit of genetic testing, over 50% of patients with rare DEEs remain undiagnosed. Sizing these patient populations and diagnosis rates underscores the importance of earlier genetic testing for unexplained seizure disorders and rare DEEs. “Even today, the path to accurate diagnosis and appropriate care for rare genetic epilepsies can be long and full of challenges,” said Adam Numis, M.D., of the UCSF Benioff Children’s Hospital. “Ambit’s methodology underscores the need for more treatment options to improve outcomes and quality of life for the rare DEE population.”
Ambit chief executive officer, Robert Sederman, will present this methodology on Oct. 13 as part of the Platform II Presentation Session (PL2-1) at the 2022 Annual Child Neurology Society Meeting. Presentations will run from 7-9 a.m. ET in the Junior Ballroom CD at the Duke Energy Center. Ambit will be presenting from 7-7:15 a.m. The associated abstract will be published in a supplement to the October issue of Annals of Neurology.
“We are excited to partner with Praxis and UCSF to showcase Ambit’s DEE projection methodology at this year’s meeting and, ultimately, see the impact of this collaboration on greater access to accurate diagnosis and treatment for patients with rare DEEs in the future,” said Sederman.
About Ambit Inc.
Ambit Inc.® is a data and technology-enabled biopharma and healthcare services company with a focus on rare and specialty disease. Ambit’s patient identification services drive patient activation and earlier diagnosis for patients that may be suffering from rare diseases. Ambit’s analytics and professional services support rare and specialty biopharma across the clinical development and commercialization of potentially life-changing therapies. To learn more about Ambit’s DEE Projection approach and other capabilities, please reach out to inquiries@ambitinc.com.
About Praxis
Praxis Precision Medicines is a clinical-stage biopharmaceutical company translating genetic insights into the development of therapies for CNS disorders characterized by neuronal excitation-inhibition imbalance. Praxis is applying insights from genetic epilepsies to both rare and more prevalent neurological disorders, using our understanding of shared biological targets and circuits in the brain. Praxis has established a broad portfolio with multiple programs, including product candidates across movement disorders, epilepsy and psychiatric disorders, with four clinical-stage product candidates. For more information, please visit www.praxismedicines.com and follow us on LinkedIn and Twitter.
Contact Information:
Alexis Burbank
Marketing, Ambit Inc.
Press Release Service
by
Newswire.com
 Tuffy Tire & Auto Service recommends AutoVitals software solutions across their network of over 160 locations.
Tuffy Tire & Auto Service recommends AutoVitals software solutions across their network of over 160 locations.
AutoVitals AutoVitals Logo
…
CEV’s patent has positioned the company to make great strides by leveraging its intellectual property to make vehicle electrification more affordable and scalable
AUSTIN, Texas – October 11, 2022 – (Newswire.com)
Current EV Motors LLC (CEV), an Austin, Texas-based vehicle electrification company, announced today that the United States Patent and Trademark Office (USPTO) issued a patent covering the application of three different “direct drive” platforms that simplify the vehicle electrification process.
ABOUT THE PATENT
The USPTO issued U.S. Patent No. 11351850B1, entitled “Universal Electric Conversion Kit for Internal Combustion Vehicles” (“the patent”), which demonstrates that through the right balance of gearing, weight calculations, vehicle dynamics, and appropriate use of battery and motor technology, a vehicle’s old transmission may be eliminated and direct drive may be implemented — optimizing the vehicle’s energy usage. CEV’s platforms based on these designs allow for a more efficient drivetrain and a faster install time, allowing EV retrofits for consumers and fleets to scale much more rapidly than traditional customer retrofits.
ABOUT CURRENT EV MOTORS
CEV was founded to simplify and scale the conversion of internal combustion vehicles to electric drive, enabling the cost-effective conversion of the world’s existing vehicle population. The founders recognized that the trends of increased classic vehicle ownership, demand for consumer and fleet vehicle electrification, and rapid development of electrification technology could be brought together in a cost-effective manner via direct drive designs. The company develops and produces kits that regional shops can install in consumer and fleet vehicles. CEV plans to open a facility in 2023 where the company will install these solutions in vehicle chassis with speed and at scale.
For media inquiries, please contact media@currentevmotors.com.
For investor inquiries, please contact ir@currentevmotors.com.
Contact Information:
Rocco Calandruccio
CEO
5125744791
Press Release Service
by
Newswire.com
Original Source:
Current EV Motors (CEV) Announces Patent Granted by USPTO
 The American Academy of Stem Cell Physicians will be hosting its fall Scientific Congress in Chicago, IL, on Oct. 28, 2022.
The American Academy of Stem Cell Physicians will be hosting its fall Scientific Congress in Chicago, IL, on Oct. 28, 2022.
AASCP Chicago lecture …
 ALBANY, N.Y. – October 11, 2022 – (Newswire.com)
ALBANY, N.Y. – October 11, 2022 – (Newswire.com)
In celebration of Cybersecurity Awareness Month, Cybersecurity pioneer, Cytex, Inc. and Mohawk Insurance announce their partnership to empower clients to protect their data assets from malicious cyberattacks and prevent data breaches with state-of-the-art DNS firewall security and Phishing Simulation.
Tom O’Connor, Mohawk Insurance Services Managing Partner, commented, “Mohawk Insurance is always looking for ways to make our clients more successful by providing added value services that protect their physical and digital assets. We chose Cytex as a partner because they’ve pioneered a comprehensive platform solution for cybersecurity that provides a high level of security, visibility, and actionable data to make our clients safer in today’s connected business environment.”
Cytex, Inc. CEO Andrew Surwilo remarked, “Remote work environments, the proliferation of connected devices, and cloud apps have created new cyber risks that can’t be defended with traditional firewalls. We are thrilled to partner with Mohawk Insurance to protect Mohawk’s clients against emerging cyber threats and data breaches.”
As small to midsize businesses continue to be the target of email phishing campaigns, Mohawk is bringing its clients a solution that increases employee cyber-threat awareness in a meaningful way. “We know that phishing campaigns targeting employee emails are one of the top ways businesses are compromised, and we recognize that the most effective way to combat this threat is to create a culture of cybersecurity awareness at the employee level through education. The Cytex Platform seamlessly combines protection, awareness, and education in an easy to implement and use dashboard,” O’Connor said.
Under the Cytex -Mohawk Insurance program, clients receive free implementation of both the DNS firewall security defense and Phishing Simulation modules within the Cytex Unified Platform for $75 per month. Mohawk is proud to offer these services and is committed to being a proactive partner in safeguarding the value its clients continue to create. To take advantage of this program, businesses can sign up at: https://cytex.io/cytex_mohawk.html
About Cytex, Inc. Cytex is a first-of-its-kind, SaaS-based platform solution that combines cybersecurity, network operations, data supply chain monitoring, and compliance automation. Cytex patent-pending technology eliminates application sprawl by combining functionality across cybersecurity and compliance domains. https://cytex.i
About Mohawk Insurance Services
Founded in 2022, Mohawk Insurance Services brings more than 100 years of combined insurance experience to service businesses, organizations, and individuals throughout New York and across the nation. We represent the leading regional and national insurance companies, providing our clients with the most comprehensive coverage, price, and service options available in the marketplace. With experienced team members in global partnerships and complex policies, we’re determined to find the best solutions for businesses big and small. https://www.mohawkinsurance.com
Contact Information:
Andrew Surwilo
CEO
703-932-8178
Tom O’Connor
Managing Partner
518-360-0030 ext. 103
Press Release Service
by
Newswire.com
Original Source:
Cytex, Inc and Mohawk Insurance Services Announce Strategic Partnership to Bring Active Cybersecurity Protection and Education to Its Clients
 The biggest maternity show of 2022 is Saturday, Oct. 22. The expo is online and free. Get tickets today. More info at happymama.global.
The biggest maternity show of 2022 is Saturday, Oct. 22. The expo is online and free. Get tickets today. More info at happymama.global.
Happy Mama …
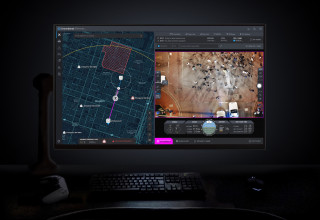
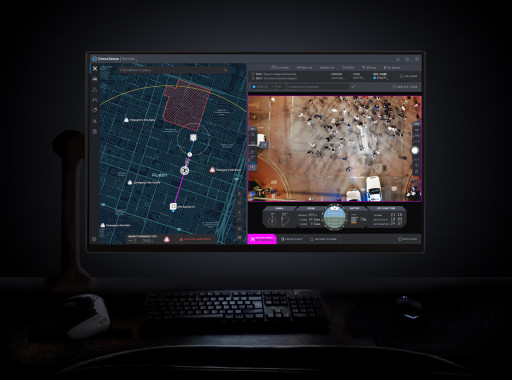 This breakthrough technology allows public safety agencies to remotely operate and deploy drones in emergencies, giving first responders a real-time view of what is happening before they arrive at the scene.
This breakthrough technology allows public safety agencies to remotely operate and deploy drones in emergencies, giving first responders a real-time view of what is happening before they arrive at the scene.

DroneSense Remote
DroneSense Remote view from the operator’s station
AUSTIN, Texas – October 11, 2022 – (Newswire.com)
DroneSense (www.dronesense.com), developers of the most trusted and reliable software platform for public safety drone operations, today announced the general availability of DroneSense Remote and Axon Air Remote powered by DroneSense. This breakthrough technology allows public safety agencies to remotely operate and deploy drones in emergencies, giving first responders a real-time view of what is happening before they arrive at the scene.
Many public safety agencies have drone programs where officers or firefighters operate a drone on scene while responding to an incident. Drone as a First Responder (DFR) programs go further, providing vital situational awareness on calls for service even before personnel arrives on scene. This approach protects both communities and public safety by giving agencies more time and information to determine the most appropriate response to a situation. The system retains detailed records of every flight, so that agencies can easily maintain transparency and accountability regarding their use of the technology within the communities they serve.
Agencies looking to start a DFR program now have everything they need for remote operations, thanks to DroneSense and Axon Air powered by DroneSense. DroneSense Remote and Axon Air Remote allow agencies to respond to any 911 call within seconds, with a single mouse click. The platform enables a teleoperator to remotely pilot drones from anywhere and aims to improve tactical decision-making and improve efficiency with fewer officer dispatches. Additionally, Axon Air Remote works seamlessly with Axon Evidence, Axon’s digital evidence management system, and Axon Respond, Axon’s real-time operations solution.
“We are excited to offer first responders the most advanced and comprehensive remote flying experience through DroneSense and Axon Air,” said Chris Eyhorn, CEO of DroneSense. “DroneSense Remote is leading the industry with innovation and support for more drone models than any other platform, allowing agencies of all sizes and budgets to increase officer safety, improve incident response time, and achieve better outcomes.”
With thousands of operational flights across leading agencies over the past few months, DroneSense Remote and Axon Air Remote have set a new bar for speed and precision in public safety remote drone operations and established an exciting new option for modern policing. The platform allows agencies to create fully-customizable flights to fit their needs, including flying DFR missions Beyond Visual Line of Sight (BVLOS) under an FAA waiver or remaining within Visual Line of Sight (VLOS) under an existing Certificate of Authorizations (COA) or Part 107 regulations. Built-in preflight checks, simple user interface, customizable geofences, continuous network integrity monitoring, and emergency landing sites create the industry’s safest approach for demanding operational environments. DroneSense Remote and Axon Air Remote even adjust for elevated takeoff locations to ensure strict adherence to designated flight altitudes.
“DroneSense Remote is the platform that has enhanced our drone program,” said Sgt. Daniel Anders with the San Antonio Police Department. “We now utilize drones to respond to calls for service much faster than we can with other assets.“
DroneSense Remote is available for the DroneSense platform, and Axon Air Remote is available for the Axon Air powered by DroneSense platform. DroneSense and Axon Air Remote applications work with public safety’s most popular drones, including the DJI Matrice 300, Mavic 2 Enterprise Dual and Zoom, and DJI’s new Matrice 30 and Mavic 3 Enterprise series drones.
To learn more about DroneSense Remote, please visit www.dronesense.com/remote or for Axon Air Remote, please visit www.axon.com/products/axon-air.
Contact Information:
Ryan Bracken
Chief Product Officer
512-582-0444
Related Images
Press Release Service
by
Newswire.com
Original Source:
DroneSense Announces General Availability of Drone as First Responder Solution for Public Safety
 Urgent Care Veterinary Clinic Redefines Patient Care Model by Bridging Gap Between Family Veterinarians and Emergency Hospitals
Urgent Care Veterinary Clinic Redefines Patient Care Model by Bridging Gap Between Family Veterinarians and Emergency Hospitals
Coming soon Vienna …